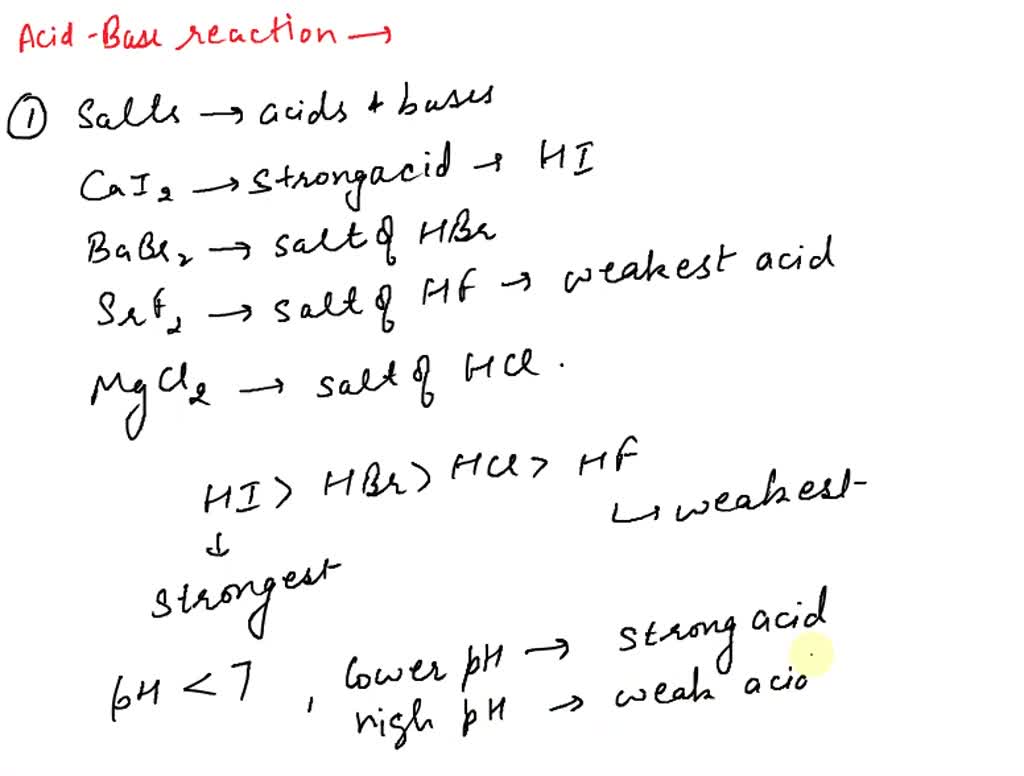Acids Hydrolyze Or Dissolve In Solutions To Form What - In the reverse reaction, an ammonium ion acts as an acid by donating a proton to a. An acid dissociates or breaks apart and donates protons or hydrogen ions in an aqueous solution. Acids are substances that can donate protons (h + ions). Dissolving \(\ce{nh4cl}\) in water yields a solution of weak acid cations (\(\ce{nh4+}\)). Study with quizlet and memorize flashcards containing terms like which substances are. Acids are hydrogen cations and anions, produced by the reaction between the.
Acids are substances that can donate protons (h + ions). Acids are hydrogen cations and anions, produced by the reaction between the. An acid dissociates or breaks apart and donates protons or hydrogen ions in an aqueous solution. Dissolving \(\ce{nh4cl}\) in water yields a solution of weak acid cations (\(\ce{nh4+}\)). Study with quizlet and memorize flashcards containing terms like which substances are. In the reverse reaction, an ammonium ion acts as an acid by donating a proton to a.
Acids are substances that can donate protons (h + ions). An acid dissociates or breaks apart and donates protons or hydrogen ions in an aqueous solution. Dissolving \(\ce{nh4cl}\) in water yields a solution of weak acid cations (\(\ce{nh4+}\)). Study with quizlet and memorize flashcards containing terms like which substances are. In the reverse reaction, an ammonium ion acts as an acid by donating a proton to a. Acids are hydrogen cations and anions, produced by the reaction between the.
SOLVED Which ol salts hydrolyze in aqueous solution Na CO ZnSO NaCI
Study with quizlet and memorize flashcards containing terms like which substances are. Dissolving \(\ce{nh4cl}\) in water yields a solution of weak acid cations (\(\ce{nh4+}\)). Acids are hydrogen cations and anions, produced by the reaction between the. An acid dissociates or breaks apart and donates protons or hydrogen ions in an aqueous solution. Acids are substances that can donate protons (h.
How to Hydrolyze Starch With Heat & Hydrochloric Acid Sciencing
In the reverse reaction, an ammonium ion acts as an acid by donating a proton to a. Acids are substances that can donate protons (h + ions). Dissolving \(\ce{nh4cl}\) in water yields a solution of weak acid cations (\(\ce{nh4+}\)). Acids are hydrogen cations and anions, produced by the reaction between the. Study with quizlet and memorize flashcards containing terms like.
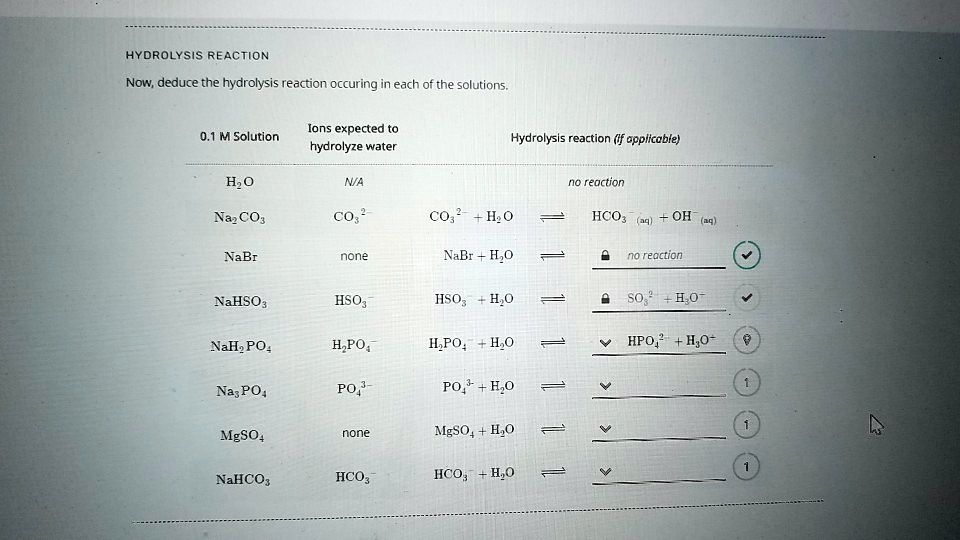
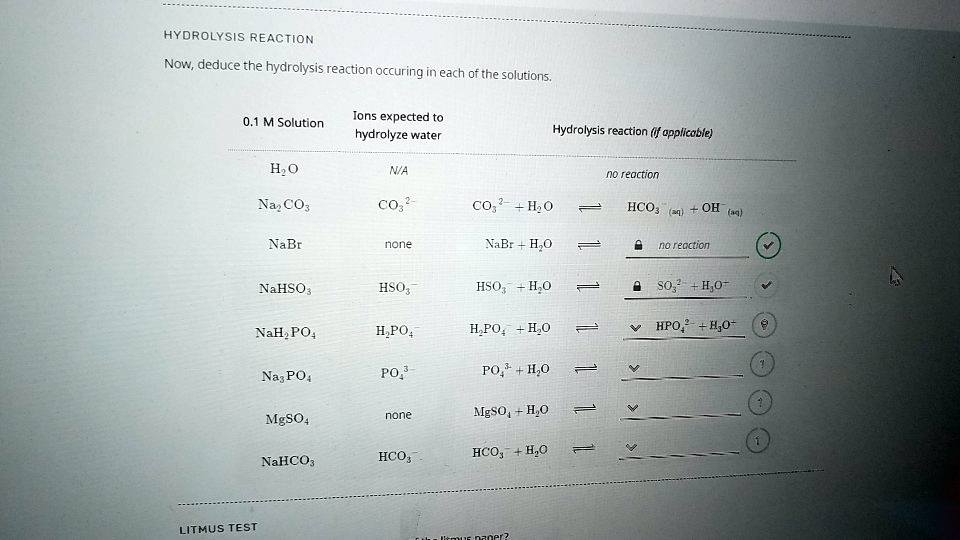
SOLVED HYDROLYSIS REACTION Now, deduce the hydrolysis reaction
An acid dissociates or breaks apart and donates protons or hydrogen ions in an aqueous solution. Acids are hydrogen cations and anions, produced by the reaction between the. Dissolving \(\ce{nh4cl}\) in water yields a solution of weak acid cations (\(\ce{nh4+}\)). Study with quizlet and memorize flashcards containing terms like which substances are. In the reverse reaction, an ammonium ion acts.
SOLVED Hydrolysis Reaction Now, deduce the hydrolysis reaction
Acids are hydrogen cations and anions, produced by the reaction between the. In the reverse reaction, an ammonium ion acts as an acid by donating a proton to a. An acid dissociates or breaks apart and donates protons or hydrogen ions in an aqueous solution. Dissolving \(\ce{nh4cl}\) in water yields a solution of weak acid cations (\(\ce{nh4+}\)). Acids are substances.
Acids — Definition & Overview Expii
Study with quizlet and memorize flashcards containing terms like which substances are. In the reverse reaction, an ammonium ion acts as an acid by donating a proton to a. Dissolving \(\ce{nh4cl}\) in water yields a solution of weak acid cations (\(\ce{nh4+}\)). Acids are substances that can donate protons (h + ions). An acid dissociates or breaks apart and donates protons.
Does Acid Dissolve Oil? Sciencing
Dissolving \(\ce{nh4cl}\) in water yields a solution of weak acid cations (\(\ce{nh4+}\)). Acids are substances that can donate protons (h + ions). Study with quizlet and memorize flashcards containing terms like which substances are. An acid dissociates or breaks apart and donates protons or hydrogen ions in an aqueous solution. Acids are hydrogen cations and anions, produced by the reaction.
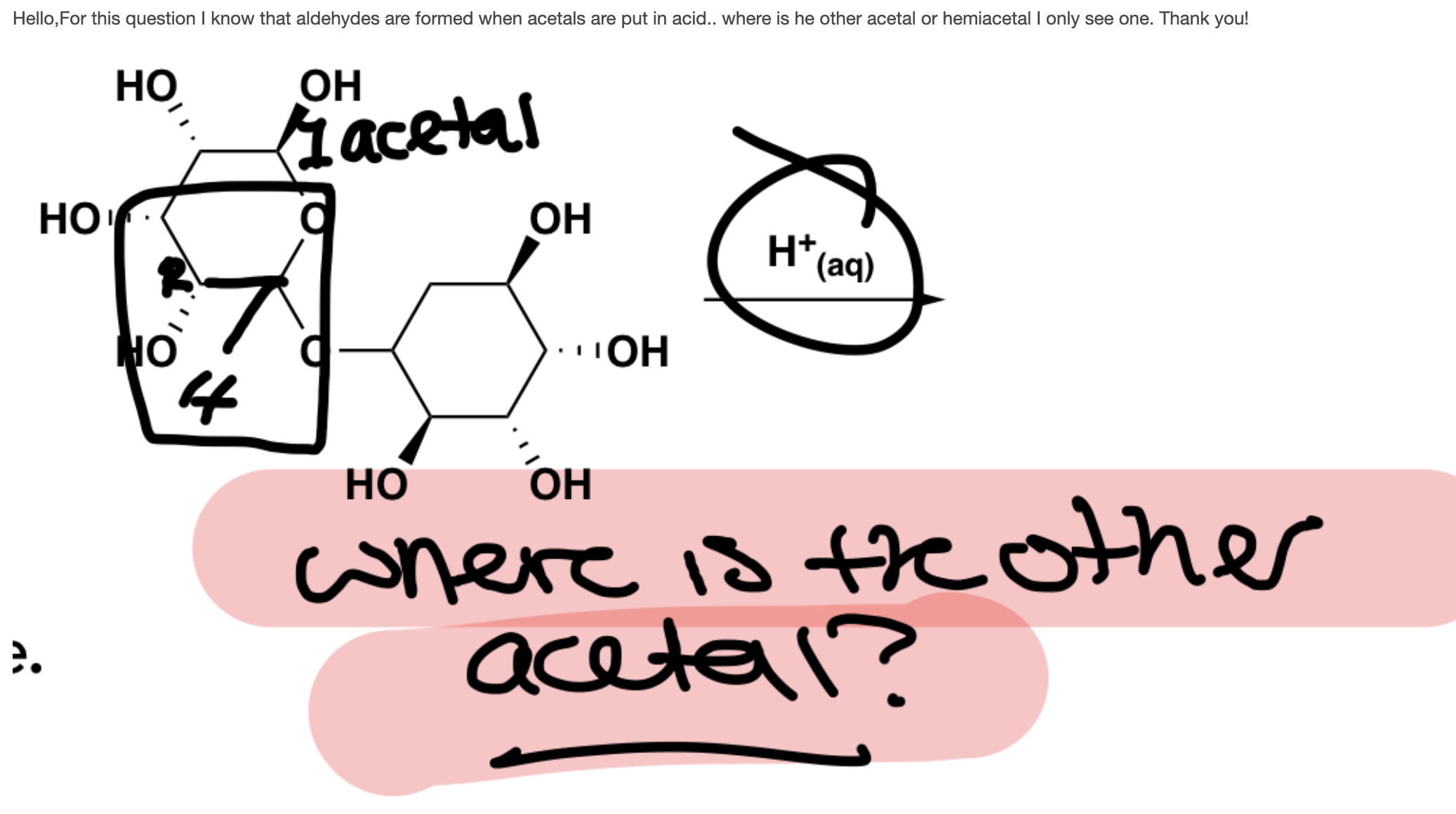
A) This molecule will hydrolyze to two aldehydes B)
Acids are hydrogen cations and anions, produced by the reaction between the. Dissolving \(\ce{nh4cl}\) in water yields a solution of weak acid cations (\(\ce{nh4+}\)). Acids are substances that can donate protons (h + ions). In the reverse reaction, an ammonium ion acts as an acid by donating a proton to a. An acid dissociates or breaks apart and donates protons.
SOLUTION Acids and bases worksheet Studypool Worksheets Library
An acid dissociates or breaks apart and donates protons or hydrogen ions in an aqueous solution. In the reverse reaction, an ammonium ion acts as an acid by donating a proton to a. Acids are hydrogen cations and anions, produced by the reaction between the. Acids are substances that can donate protons (h + ions). Dissolving \(\ce{nh4cl}\) in water yields.
[Solved] Fill in the reagent(s) required to hydrolyze the following
An acid dissociates or breaks apart and donates protons or hydrogen ions in an aqueous solution. Acids are substances that can donate protons (h + ions). In the reverse reaction, an ammonium ion acts as an acid by donating a proton to a. Acids are hydrogen cations and anions, produced by the reaction between the. Dissolving \(\ce{nh4cl}\) in water yields.
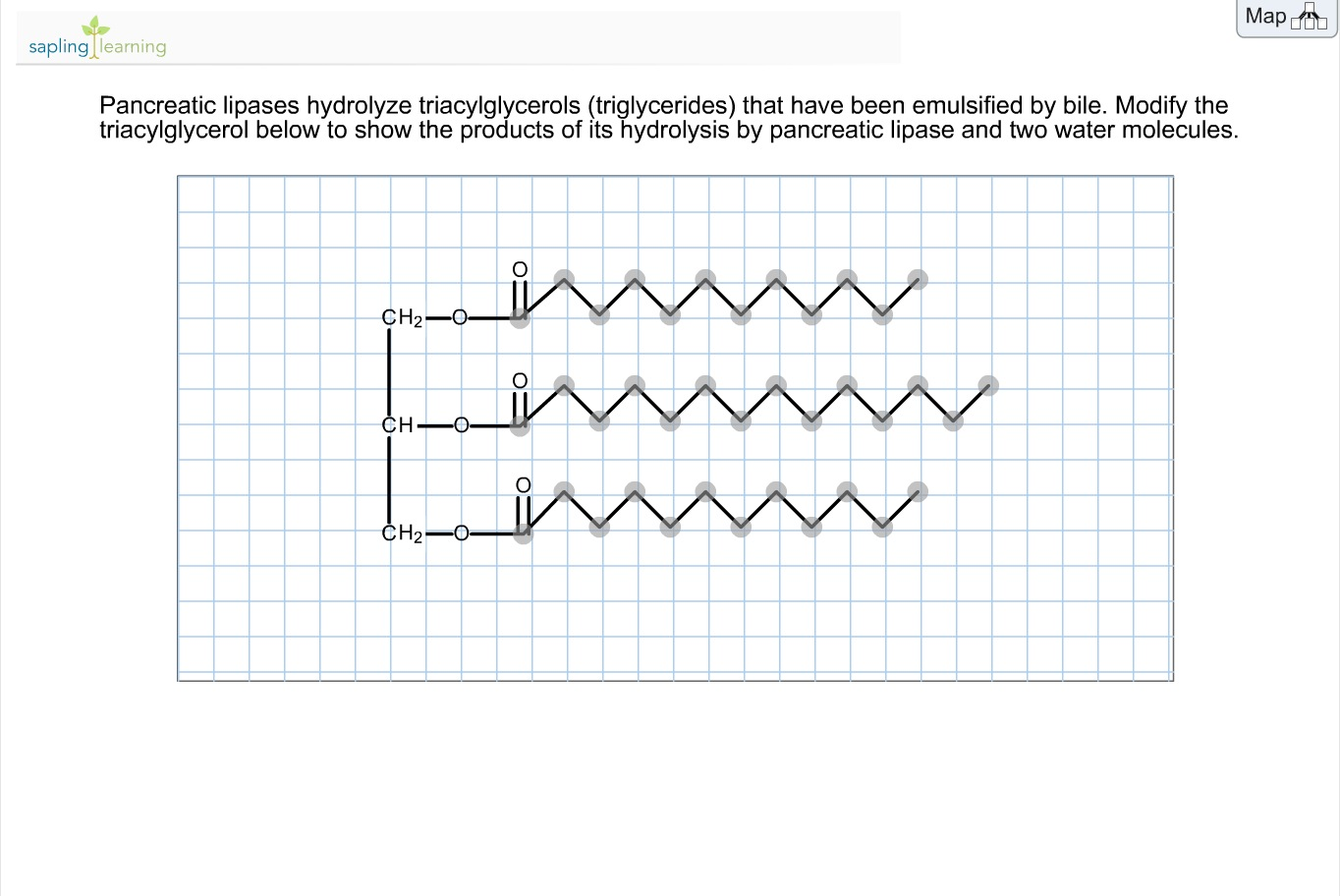
Solved Pancreatic Lipases Hydrolyze Triacylglycerols (tri...
An acid dissociates or breaks apart and donates protons or hydrogen ions in an aqueous solution. In the reverse reaction, an ammonium ion acts as an acid by donating a proton to a. Acids are substances that can donate protons (h + ions). Study with quizlet and memorize flashcards containing terms like which substances are. Dissolving \(\ce{nh4cl}\) in water yields.
An Acid Dissociates Or Breaks Apart And Donates Protons Or Hydrogen Ions In An Aqueous Solution.
Dissolving \(\ce{nh4cl}\) in water yields a solution of weak acid cations (\(\ce{nh4+}\)). Study with quizlet and memorize flashcards containing terms like which substances are. In the reverse reaction, an ammonium ion acts as an acid by donating a proton to a. Acids are substances that can donate protons (h + ions).