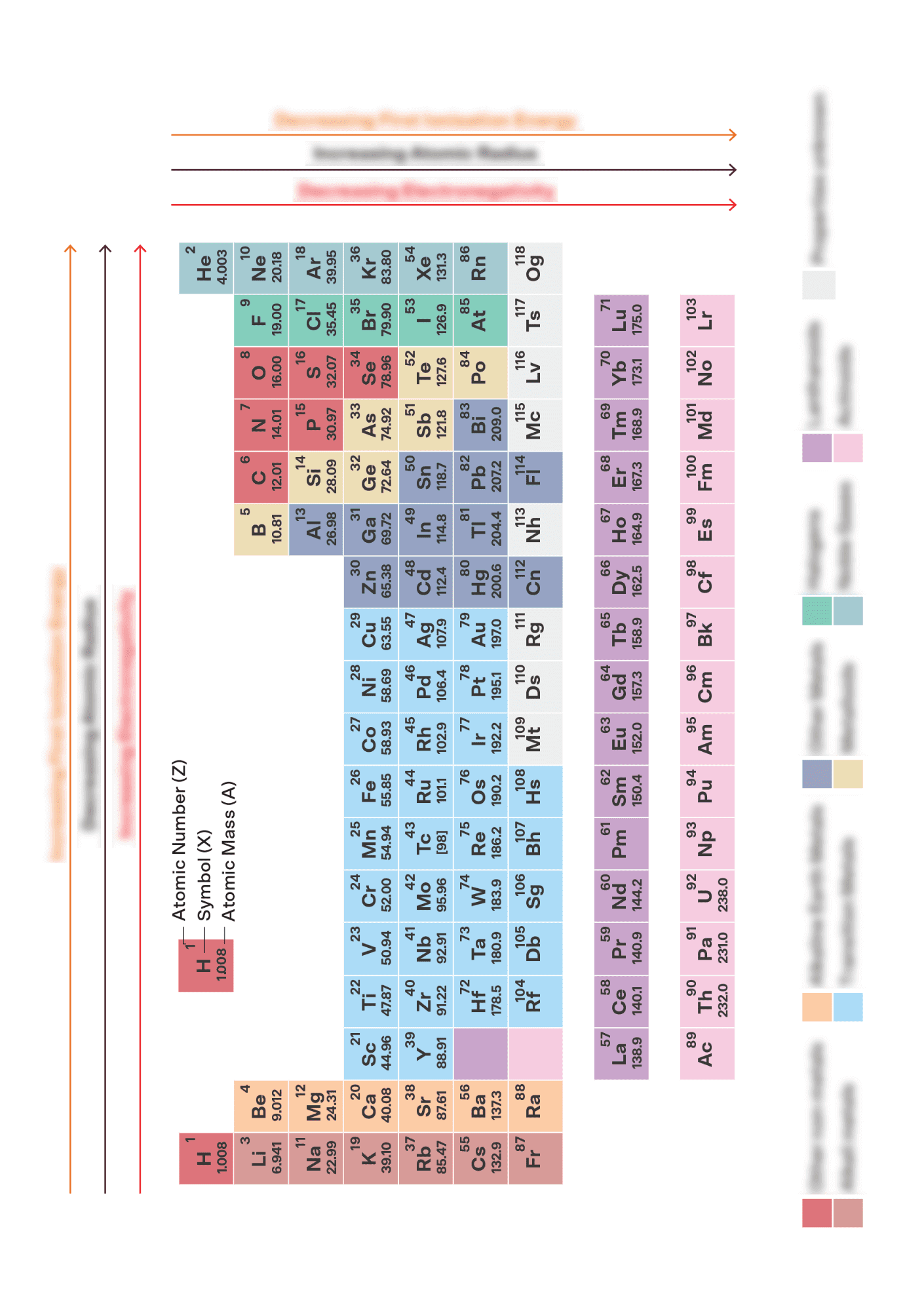
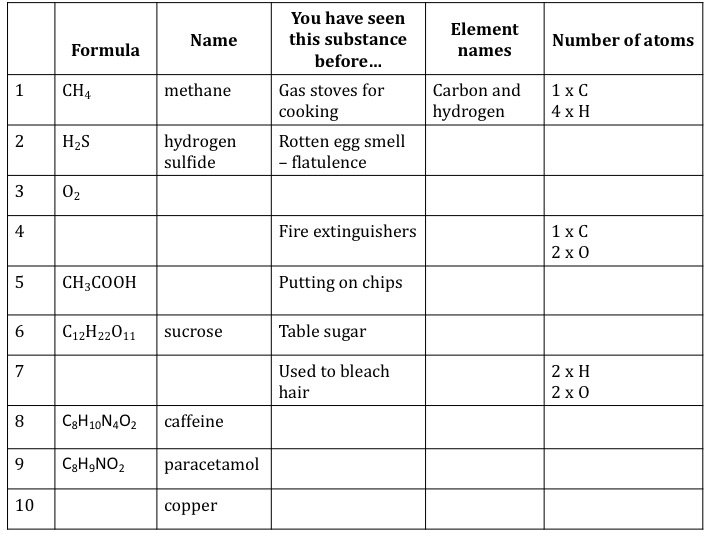
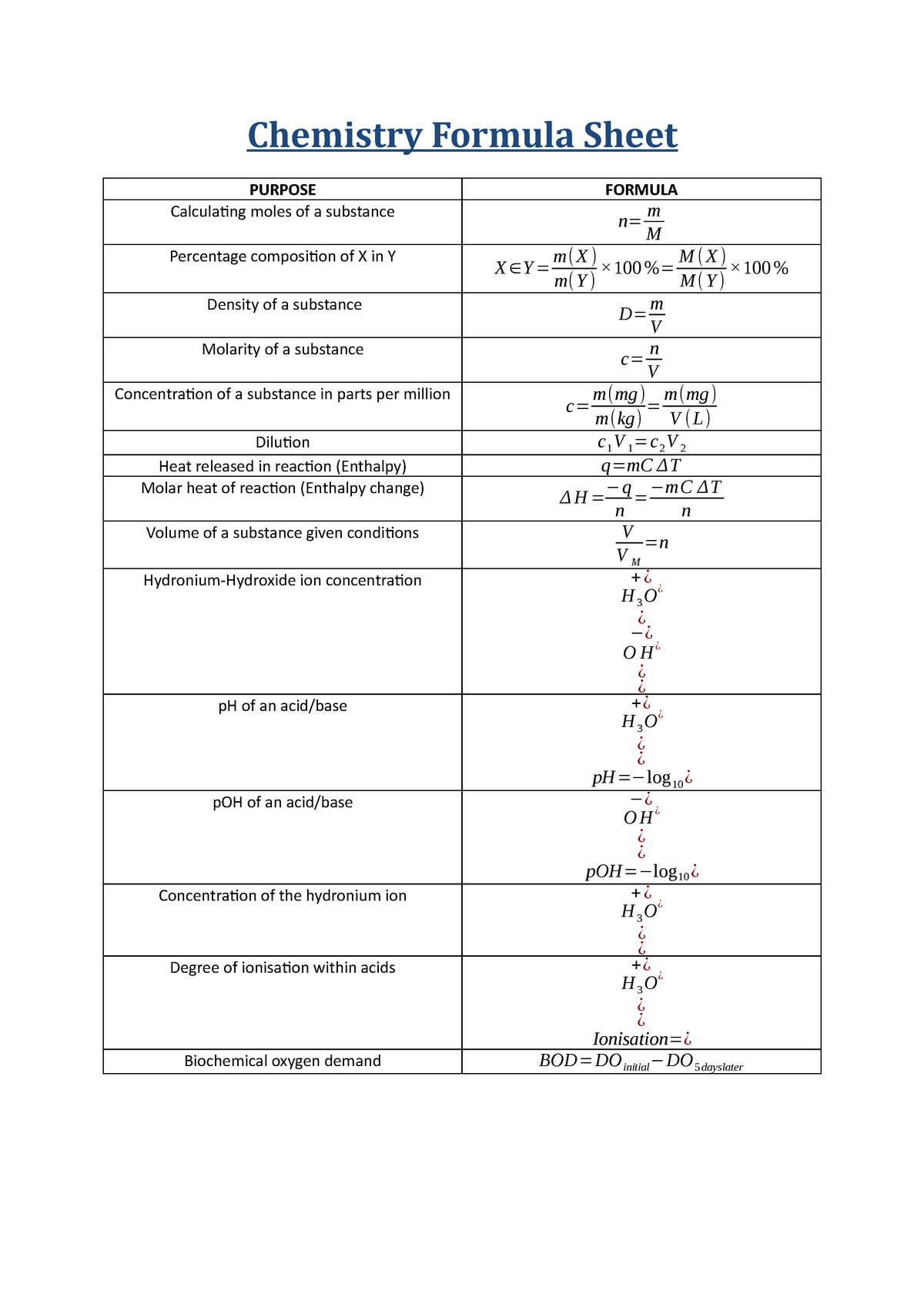
Chemical Formula Sheet For Chemistry - Initial reaction between lead and hydrochloric acid produce lead (ii) chloride or lead (ii) sulfate which is insoluble. A chemical formula is a way through which scientists represent the chemical proportions such as the number of atoms present in a. *carbon (c) and hydrogen gas (h2) added for comparison. It will form a coating around metal. Chemistry formula and constant sheet and periodic table created date: Characteristic range of infrared absorption due to stretching in organic molecules.
Chemistry formula and constant sheet and periodic table created date: Characteristic range of infrared absorption due to stretching in organic molecules. A chemical formula is a way through which scientists represent the chemical proportions such as the number of atoms present in a. Initial reaction between lead and hydrochloric acid produce lead (ii) chloride or lead (ii) sulfate which is insoluble. *carbon (c) and hydrogen gas (h2) added for comparison. It will form a coating around metal.
Chemistry formula and constant sheet and periodic table created date: Characteristic range of infrared absorption due to stretching in organic molecules. *carbon (c) and hydrogen gas (h2) added for comparison. It will form a coating around metal. Initial reaction between lead and hydrochloric acid produce lead (ii) chloride or lead (ii) sulfate which is insoluble. A chemical formula is a way through which scientists represent the chemical proportions such as the number of atoms present in a.
Chemical Formula Chart Chemical Formula Info
Chemistry formula and constant sheet and periodic table created date: A chemical formula is a way through which scientists represent the chemical proportions such as the number of atoms present in a. *carbon (c) and hydrogen gas (h2) added for comparison. Characteristic range of infrared absorption due to stretching in organic molecules. It will form a coating around metal.
Chemistry Formula Cheat Sheet
A chemical formula is a way through which scientists represent the chemical proportions such as the number of atoms present in a. *carbon (c) and hydrogen gas (h2) added for comparison. Chemistry formula and constant sheet and periodic table created date: Initial reaction between lead and hydrochloric acid produce lead (ii) chloride or lead (ii) sulfate which is insoluble. Characteristic.
Chemical Formulas List For Class 10 PDF Hydroxide Oxide
Initial reaction between lead and hydrochloric acid produce lead (ii) chloride or lead (ii) sulfate which is insoluble. It will form a coating around metal. Chemistry formula and constant sheet and periodic table created date: *carbon (c) and hydrogen gas (h2) added for comparison. Characteristic range of infrared absorption due to stretching in organic molecules.
Naming Formulas Chemistry Calculator
Chemistry formula and constant sheet and periodic table created date: A chemical formula is a way through which scientists represent the chemical proportions such as the number of atoms present in a. *carbon (c) and hydrogen gas (h2) added for comparison. Initial reaction between lead and hydrochloric acid produce lead (ii) chloride or lead (ii) sulfate which is insoluble. Characteristic.
Chemistry Formulas Cheat Sheet Chemistry education, Chemistry notes
*carbon (c) and hydrogen gas (h2) added for comparison. Chemistry formula and constant sheet and periodic table created date: Initial reaction between lead and hydrochloric acid produce lead (ii) chloride or lead (ii) sulfate which is insoluble. Characteristic range of infrared absorption due to stretching in organic molecules. A chemical formula is a way through which scientists represent the chemical.
Naming Of Chemical Formula
Characteristic range of infrared absorption due to stretching in organic molecules. Initial reaction between lead and hydrochloric acid produce lead (ii) chloride or lead (ii) sulfate which is insoluble. *carbon (c) and hydrogen gas (h2) added for comparison. It will form a coating around metal. A chemical formula is a way through which scientists represent the chemical proportions such as.
Writing chemical formulae teaching resources the science teacher
*carbon (c) and hydrogen gas (h2) added for comparison. Initial reaction between lead and hydrochloric acid produce lead (ii) chloride or lead (ii) sulfate which is insoluble. It will form a coating around metal. A chemical formula is a way through which scientists represent the chemical proportions such as the number of atoms present in a. Chemistry formula and constant.
Naming Formulas Chemistry Calculator
*carbon (c) and hydrogen gas (h2) added for comparison. Initial reaction between lead and hydrochloric acid produce lead (ii) chloride or lead (ii) sulfate which is insoluble. It will form a coating around metal. A chemical formula is a way through which scientists represent the chemical proportions such as the number of atoms present in a. Chemistry formula and constant.
Formula Sheet Level 2 Chemistry at Alfred Ma blog
Chemistry formula and constant sheet and periodic table created date: A chemical formula is a way through which scientists represent the chemical proportions such as the number of atoms present in a. Initial reaction between lead and hydrochloric acid produce lead (ii) chloride or lead (ii) sulfate which is insoluble. It will form a coating around metal. *carbon (c) and.
Chemistry Cheat Sheet Printable
Chemistry formula and constant sheet and periodic table created date: *carbon (c) and hydrogen gas (h2) added for comparison. A chemical formula is a way through which scientists represent the chemical proportions such as the number of atoms present in a. Initial reaction between lead and hydrochloric acid produce lead (ii) chloride or lead (ii) sulfate which is insoluble. It.
It Will Form A Coating Around Metal.
Initial reaction between lead and hydrochloric acid produce lead (ii) chloride or lead (ii) sulfate which is insoluble. Characteristic range of infrared absorption due to stretching in organic molecules. A chemical formula is a way through which scientists represent the chemical proportions such as the number of atoms present in a. *carbon (c) and hydrogen gas (h2) added for comparison.