How Do Hydrogen Bonds Form Between Water Molecules - Hydrogen bonds form between water molecules when the partially positive end of. Identify three special properties of water that make it unusual for a molecule of its. The covalent bonds of water make it a polar molecule.
Identify three special properties of water that make it unusual for a molecule of its. The covalent bonds of water make it a polar molecule. Hydrogen bonds form between water molecules when the partially positive end of.
Identify three special properties of water that make it unusual for a molecule of its. Hydrogen bonds form between water molecules when the partially positive end of. The covalent bonds of water make it a polar molecule.
Are Hydrogen Bonds The Strongest Interactions Between Molecules?
The covalent bonds of water make it a polar molecule. Identify three special properties of water that make it unusual for a molecule of its. Hydrogen bonds form between water molecules when the partially positive end of.
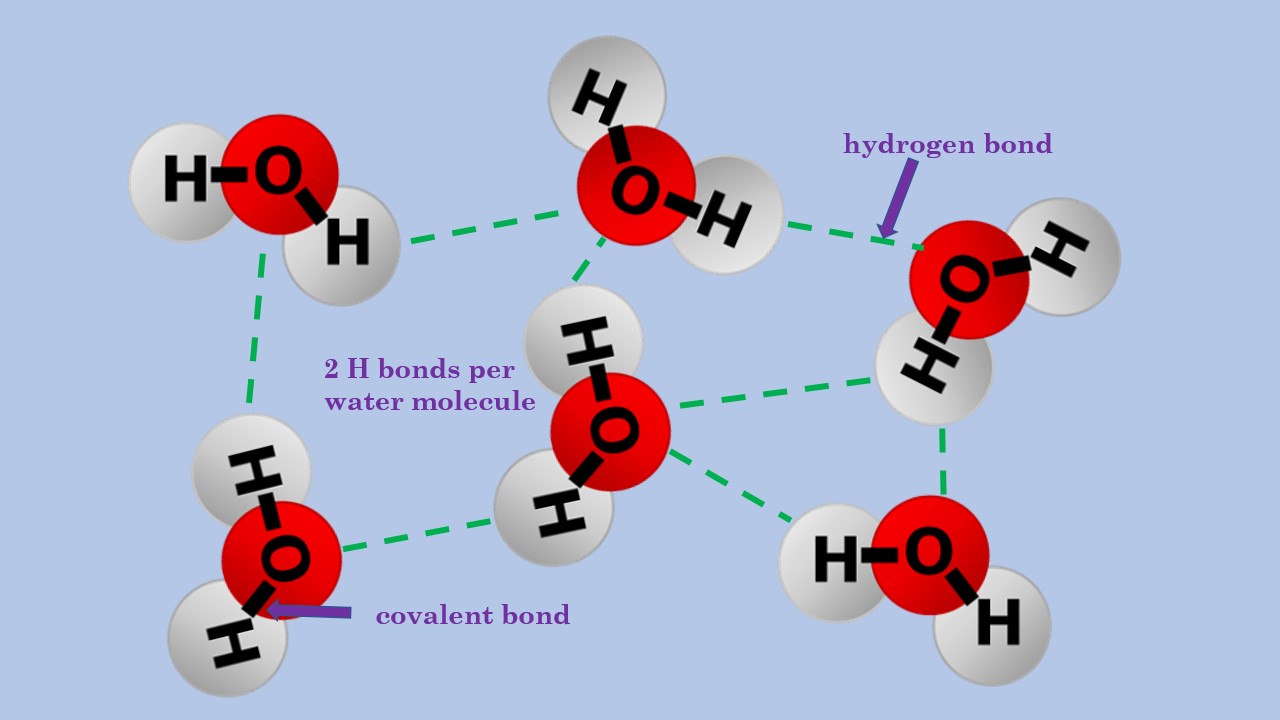
Water Molecules And Hydrogen Bonding Diagram
The covalent bonds of water make it a polar molecule. Identify three special properties of water that make it unusual for a molecule of its. Hydrogen bonds form between water molecules when the partially positive end of.
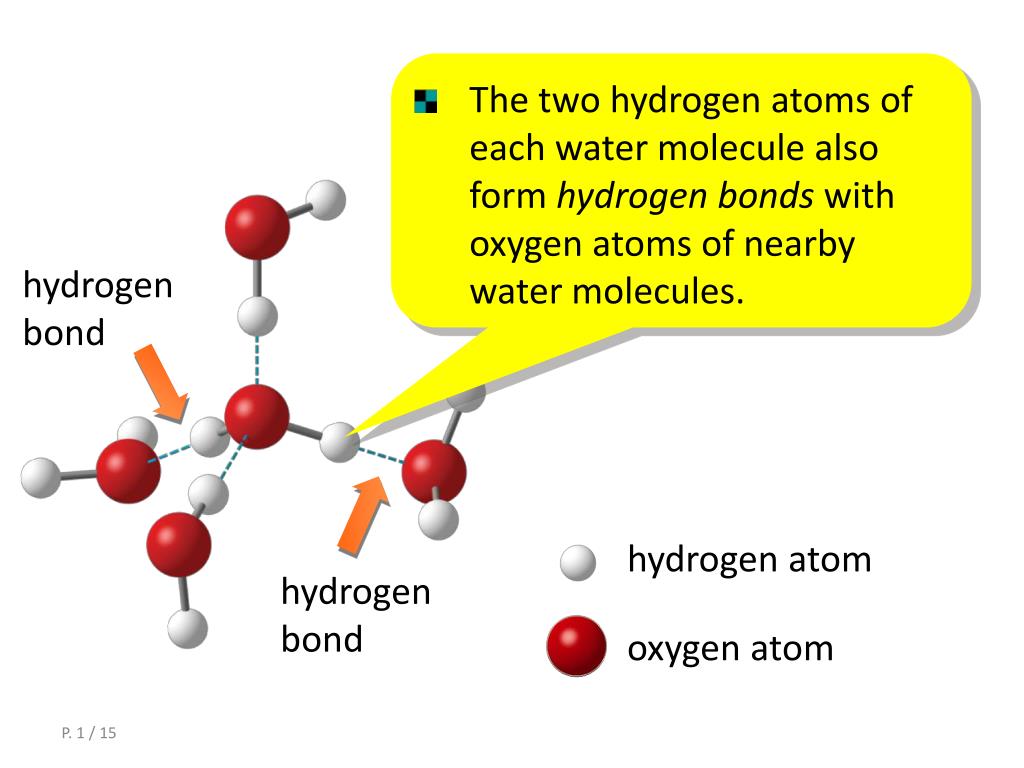
Draw A Hydrogen Bond Between Two Water Molecules
Identify three special properties of water that make it unusual for a molecule of its. The covalent bonds of water make it a polar molecule. Hydrogen bonds form between water molecules when the partially positive end of.
Diagram of hydrogen bonding between two water molecules Download
Identify three special properties of water that make it unusual for a molecule of its. The covalent bonds of water make it a polar molecule. Hydrogen bonds form between water molecules when the partially positive end of.
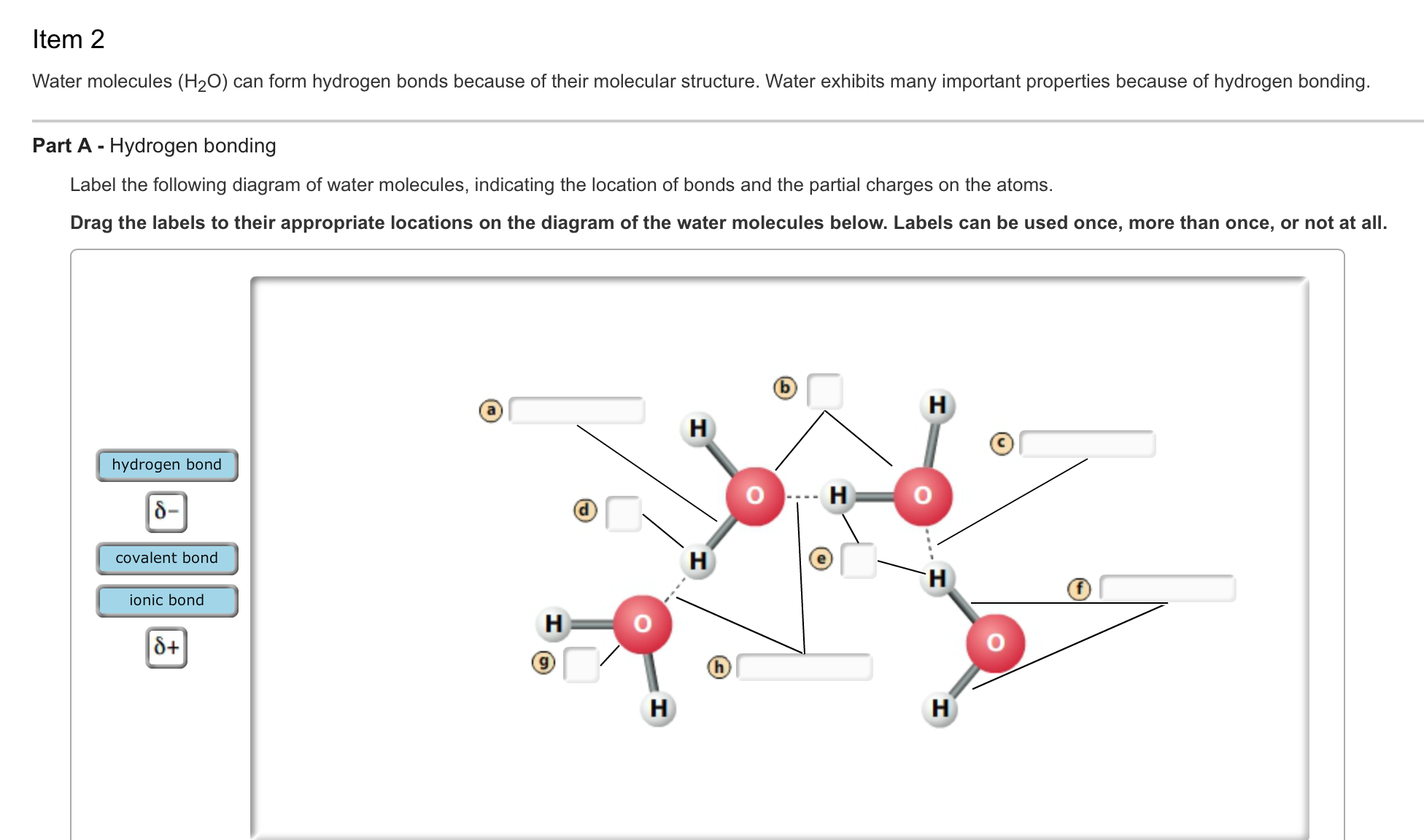
Solved Water molecules (H_2O) can form hydrogen bonds
Identify three special properties of water that make it unusual for a molecule of its. The covalent bonds of water make it a polar molecule. Hydrogen bonds form between water molecules when the partially positive end of.
hydrogen bond between water molecules Diagram Quizlet
Hydrogen bonds form between water molecules when the partially positive end of. Identify three special properties of water that make it unusual for a molecule of its. The covalent bonds of water make it a polar molecule.
Draw A Diagram Of Water Molecules Labeling The Hydrogen Bond And
The covalent bonds of water make it a polar molecule. Identify three special properties of water that make it unusual for a molecule of its. Hydrogen bonds form between water molecules when the partially positive end of.
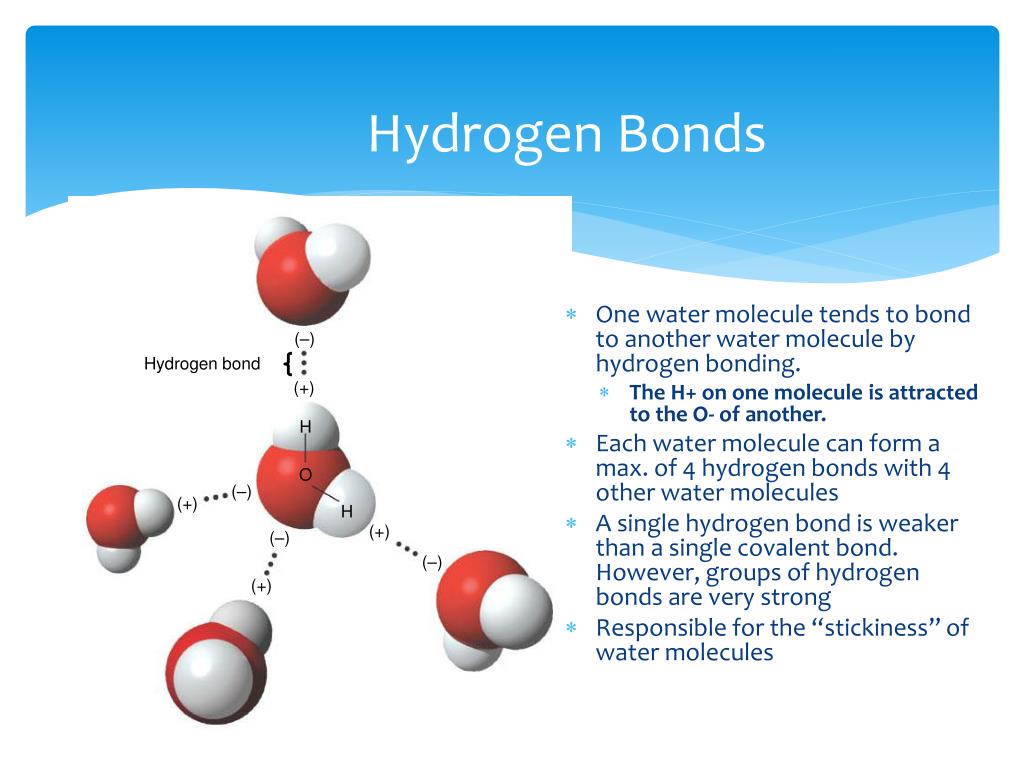
Water Molecules Hydrogen Bonds
Identify three special properties of water that make it unusual for a molecule of its. Hydrogen bonds form between water molecules when the partially positive end of. The covalent bonds of water make it a polar molecule.
Water Molecules Hydrogen Bonds
Hydrogen bonds form between water molecules when the partially positive end of. Identify three special properties of water that make it unusual for a molecule of its. The covalent bonds of water make it a polar molecule.
The Covalent Bonds Of Water Make It A Polar Molecule.
Identify three special properties of water that make it unusual for a molecule of its. Hydrogen bonds form between water molecules when the partially positive end of.